Were You Diagnosed With A Brain Tumor After Taking Depo-Provera? Van Law Firm May Be Able To Help
Overview of Birth Control Options
When it comes to birth control, American women have several options. They can take pills, use patches, or internal devices to regulate various hormones and help prevent pregnancy. Another option is taking a so-called “birth control shot.”
U.S. doctors treating women who prefer the latter typically prescribe one of two medroxyprogesterone acetate shots available here under the brand names Depo-Provera or Depo-SubQ Provera 104. Experts say these shots are highly effective and provide protection for three months at a time, making them a viable alternative for women who don’t want to take daily pills.
For a free legal consultation, call (725) 900-9000
Depo-Provera and Known Side Effects
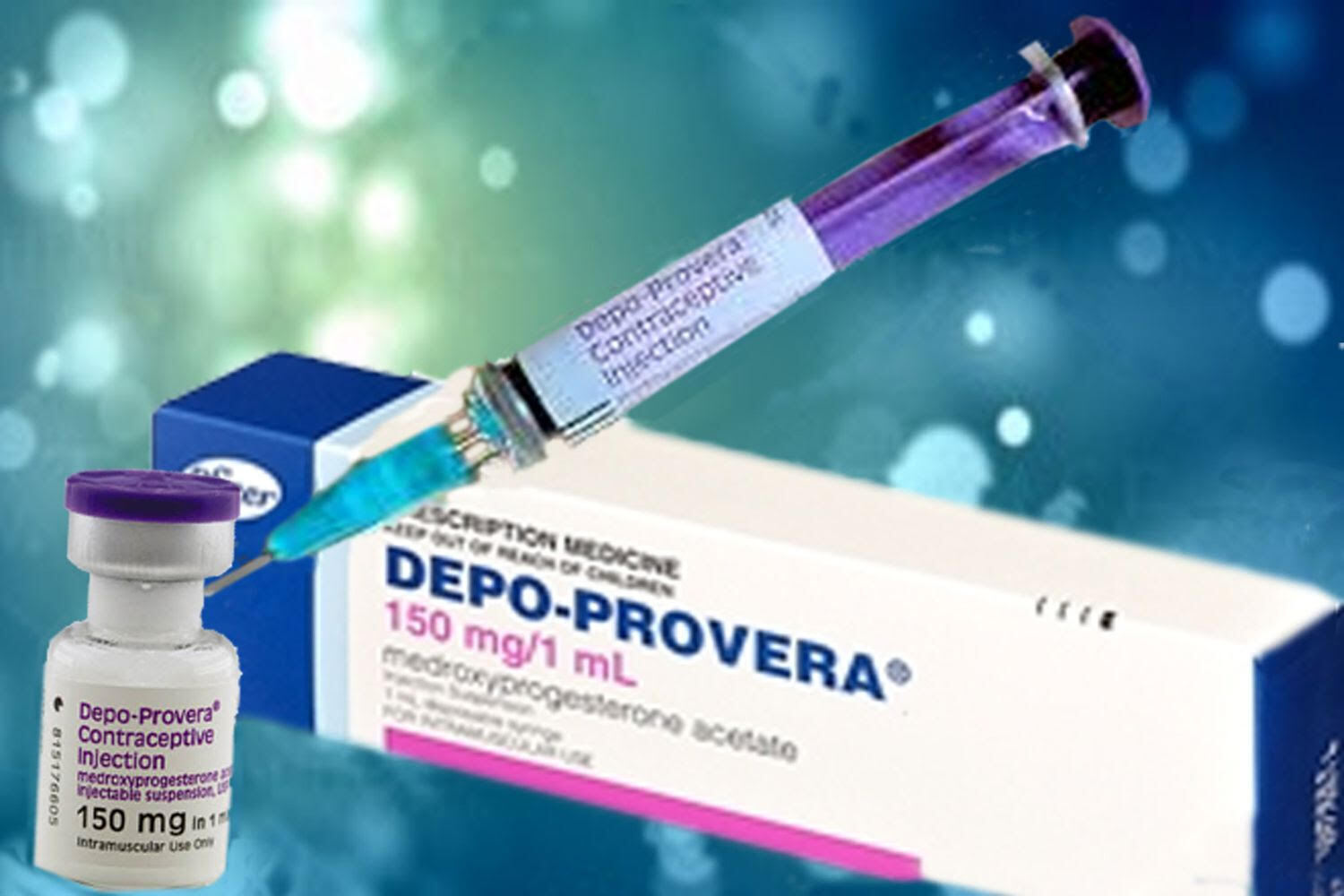
Of course, there are always risks. Known side effects associated with Depo-Provera range from minor to severe. Examples include but are not limited to abdominal pain, weight gain, mood changes, headaches, blood clots, major depression, and bone density loss potentially leading to osteoporosis. Of particular relevance here is a recent study that linked Depo-Provera use to meningioma, a type of brain tumor.
If you or a family member has been diagnosed with meningioma after using Depo-Provera, Van Law Firm may be able to help. Keep reading to learn more.
What Is Depo-Provera And How Does It Work?
As summarized above, Depo-Provera is an injection administered in the buttocks or shoulder muscle four times per year to help prevent pregnancy. Classified as a “progestin” or synthetic version of the hormone progesterone, Depo-Provera works by maintaining stable progesterone levels. This halts the menstrual cycle and ultimately ovulation.
Pfizer, the drug company responsible for developing and patenting Depo-Provera, received U.S. Food and Drug Administration (FDA) approval for use over 30 years ago. It has since been available in generic versions, many still made by Pfizer and sold by other companies.
Click to contact our personal injury lawyers today
Pfizer’s Responsibility in Depo-Provera Product Liability Cases

Because Pfizer manufactures Depo-Provera, it is legally obligated to ensure it is safe and to warn users about any risks and side effects associated with its use. On its company website, Pfizer informs patients about certain health risks and side effects associated with the drug. Specifically, Pfizer warns women not to take it if they:
- Have had unexplained vaginal bleeding
- Have or suspect breast cancer
- Have had a stroke
- Have a history of blood clots in their extremities or lungs
- Have liver disease or other ailments affecting the liver
- Are allergic to medroxyprogesterone acetate or any of the other ingredients in Depo-Provera
Pfizer also instructs patients to inform their healthcare providers about certain health conditions before taking Depo-Provera, and it lists various side effects associated with its use. However, Pfizer is now accused of failing to include meningioma as a potential health risk in its warning label, thereby violating its duty to users.
Complete a Free Case Evaluation form now
Identifying The Link Between Depo-Provera and Meningioma
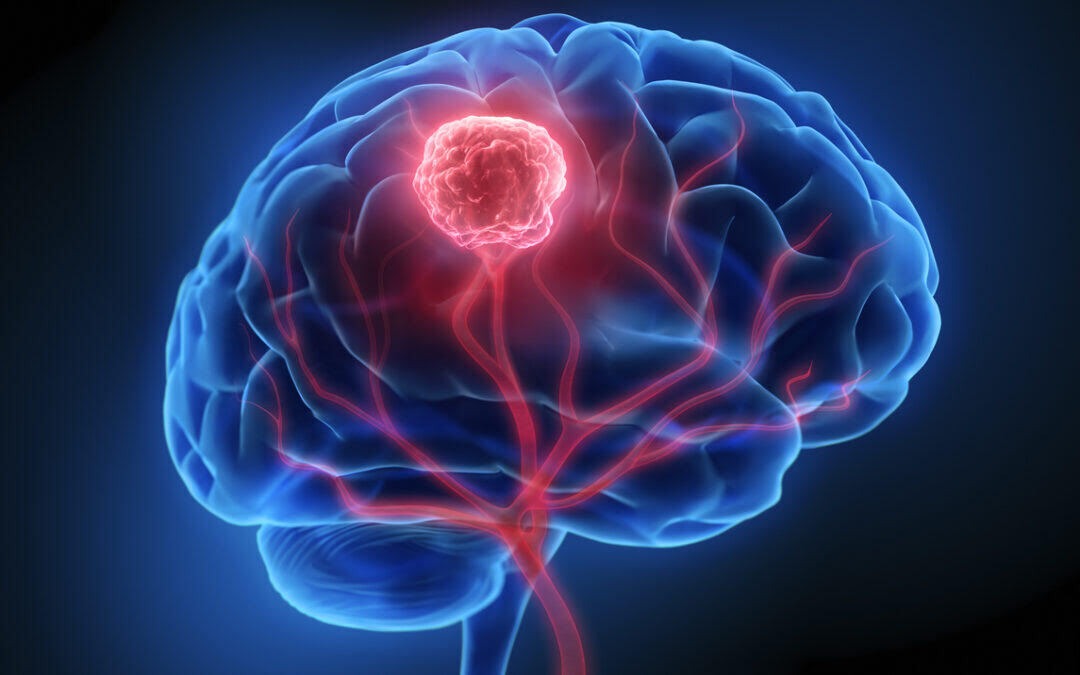
A recent study identified a link between Depo-Provera use and meningioma occurrences. The British Medical Journal (BMJ) published the study in March 2024, conducted by French researchers. The study explored potential ties between the use of certain medications classified as progestogens and the risks of developing meningioma.
These medications included:
- Progesterone
- Hydroxyprogesterone
- Dydrogesterone
- Medrogestone
- Medroxyprogesterone acetate
- Promegestone
- Dienogest
- Intrauterine levonorgestrel
The study, which included over 108,000 participants (18,061 women who had intracranial surgery for meningioma between 2009 and 2018), found that prolonged use of medrogestone, medroxyprogesterone acetate, and promegestone increased the risk of intracranial meningioma. The heightened risk associated with injectable medroxyprogesterone acetate, a widely used contraceptive, was one of the critical findings.
What You Need To Know About Meningioma
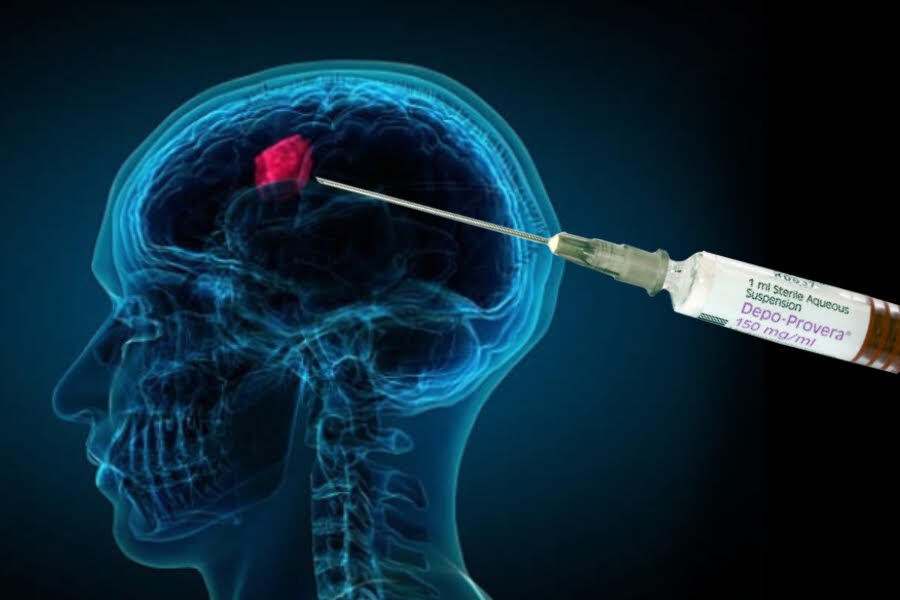
According to the Mayo Clinic, a meningioma is a tumor that grows from the membranes surrounding the brain and spinal cord, called the meninges. It is the most common type of tumor that develops in the head and is more likely to occur in women.
Meningiomas are characterized by slow growth and don’t necessarily require immediate treatment. Monitoring may be recommended instead. Symptoms can include:
- Vision abnormalities, such as seeing double or blurring
- Headaches that are worse in the morning
- Difficulty hearing or ringing in the ears
- Memory loss
- Loss of smell
- Seizures
- Weakness in the extremities
- Trouble speaking
Experts recommend seeking immediate medical attention if you experience seizures or sudden changes in vision or memory. If symptoms worsen over time, schedule an appointment with your healthcare provider.
Contact Van Law Firm If You Took Depo-Provera And Were Diagnosed With Meningioma
If you or a loved one were diagnosed with meningioma after taking Depo-Provera, you may have options for legal recourse. The personal injury attorneys at Van Law Firm can assess your circumstances to determine if you have a viable case. If you do, we’ll explain your legal options, including potential inclusion in a class action lawsuit. Contact us today to schedule a consultation.
No obligation consultations are always free.
Let Us Help You! Call Now: (725) 900-9000
